Pharmaceutical and legal experts in local regulations.
Private offices in each country with dedicated personnel, not contractors.
Focus on Regulatory Excellence and Compliance.
Expertise in the registration of advanced therapy products.
You hire know-how and deep knowledge experts in local regulations and requirements for new products registration.
Step ahead your regulatory results with standardized processes, technological support and strong commitment.
Optimize in advance the renewal strategy with changes if applicable.
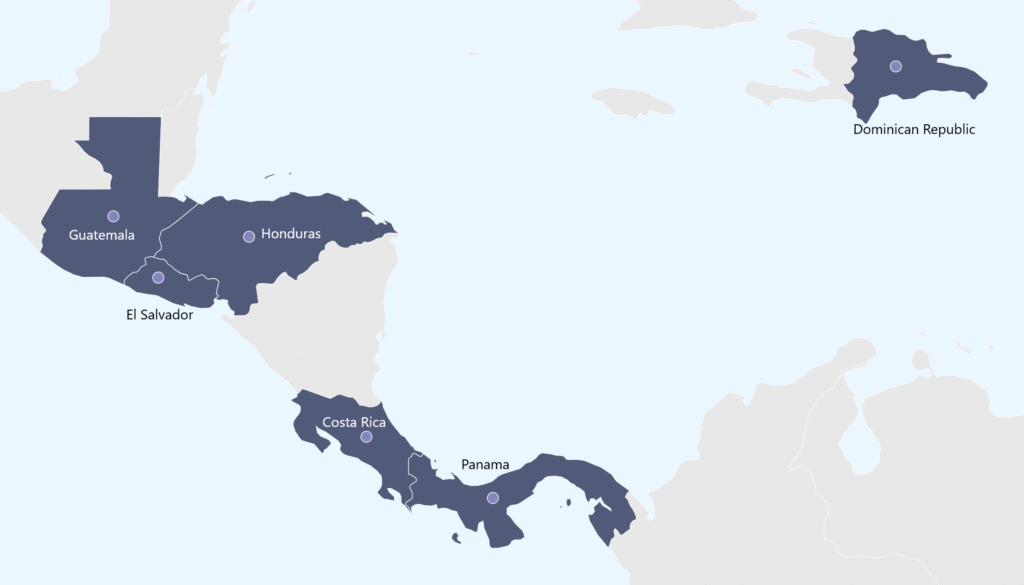
Registrek has its own offices and provides regulatory affairs services in several countries.
Powering regulatory access for drugs, medical devices, cosmetics and advanced therapies.
Our clients inspire us every day, each market approval is our celebration.
Every regulatory process is a commitment.
Regulatory Intelligence is our direction to have advanced expertise in service execution.
Integrated knowledge and effective management of self-standards provides Registrek with a successful product lifecycle of human health products.
at a glance
Corporate Governance is a mandatory element for Registrek success in a regulated environment.
Our technical integrated capabilities and our software technology convey into a specific follow-up of the product status at all times.
Compliance is a word that Registrek emphasizes daily, we focus on every step of the way to minimize potential impact of product to market delays, considering the challenges of a regulatory environment, that our industry faces every day.
Empower your company results with regulatory strategy.